The Cavender Laboratory!
Research and discovery are at the heart of scientific learning. There is no greater sense of accomplishment than designing an experiment to investigate a captivating problem, then effectively performing that experiment, collecting data and discovering something new! The Cavender lab has several ongoing research projects that students directly engage in to learn techniques, experimental design, data analysis and presentation. The goal is to develop students into independent research scientists who are prepared for graduate school or an immediate career in the biomedical sciences.
Ongoing Projects in the Lab
The Cavender lab utilizes the simian virus 40 oncovirus as a model to study cellular transformation initiation and progression. Our main focus is investigating the Large T (tumor) antigen which is an oncoprotein capable of fully transforming rodent cells. This model is relevant and all data generated can be applied to the literature of DNA tumor viruses.
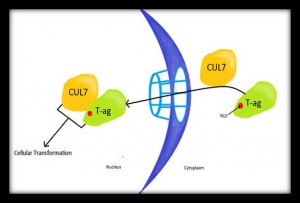
Cul7 and SV40 T antigen:This research proposal is the brainchild of senior Sarah Sulon ’14. Sarah is interested in discovering the relationship between T antigen and the E3 ubiquitin ligase, Cullin 7 (Cul7). Cul7 was discovered as a cellular protein bound to T-antigen. In normal cells, Cul7 functions in development through mono-ubiquination signaling. If poly-ubiquination of four or more ubiquitin units are attached at the lysine 48 residue of a peptide, this signals for degradation of the protein by the proteasome. The Cul7 binding to T antigenhas been found to be essential in the full cellular transformation process by SV40. Despite efforts through immunofluorescence to decipher where in the cell the T:Cul7 complex forms, concentration of the complex are too low to detect through this method. As a result, the literature establishes the trafficking patterns of this complex to be a point of interest for future research. Recent research has also posed the question of the involvement of Cul7 ubiquination activity in the cellular transformation induced by SV40 T antigen. Our lab intends to determine the localization of the T-ag/Cul7 complex in C57 Black 6 mouse embryo fibroblast (B6MEF) cells expressing either wild type T antigen or a nuclear localization deficient mutant.
2. SV40 T antigen transactivation of Cyclins A and E and the relationship to the ribosomal promoter activation

Kyle Lord ’13
Kyle Lord ’13 studied the capabilities of T antigen mutants to transactivate the cyclin A and ribosomal promoters, hypothesizing that increased expression of the cyclin and subsequent binding to its kinase partners would result in phosphorylation of key transcription factors for rDNA. For transcription of ribosomal genes to occur, upstream binding factor (UBF) must be phosphorylated on Ser388; an activity known to be accomplished by either cyclin A, E or both. This study focused on the central portion of T antigen spanning amino acids 351 to 708, which includes the p53 binding domain. Preliminary data has shown that although the N-terminal activities of T antigen seem to be necessary for the transactivation of both the ribosomal and cyclin A promoters, the central portion, including the p53 binding region, appears to be only a requirement for the ribosomal- but not cyclin A-, transactivation.
3. SV40 T antigen Inhibition of Differentiation
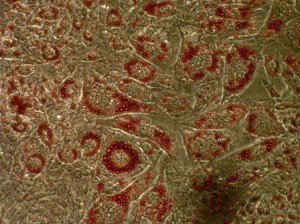
The Cavender lab also has an interest in what activities of the tumor protein SV40 T antigen are necessary to block the transition from a stem cell into a fully differentiated adipocyte (fat cell). The analogy can be made that the processes of tumorigenesis and differentiation are opposite sides of the same coin: the tumor cell pushing the cell to divide and the differentiated cell shutting down the pathways that lead to replication. This project extends the findings by Higgins (1996) that showed differentiation of pre-adipocytes into fat producing cells can be functionally blocked by the viral oncoprotein SV40 T antigen. Specifically, in that study differentiation was reliant upon functional retinoblastoma gene product and an unknown activity marked by the amino acids 121-708 in T antigen. We postulated that this activity was the p53 tumor suppressor protein. To test this hypothesis we established cell lines stably expressing T antigen with mutations in the p53- binding region as well lines expressing T antigen with mutations in both the Rb- and p53- binding regions. Additionally, cell lines were established expressing the N- terminal fragment of T antigen bearing J-domain mutations in order to assess the need for ATPase function in differentiation blockage. Seven days after differentiation was induced, the cells were stained and examined for phenotypic changes characteristic of mature adipocytes. While wild type pre-adipocytes readily differentiated under these conditions, all lines containing T antigen or its mutants showed little to no signs of differentiation.